By Steve Kirsch
None of the patients followedA new study from Harvard (Continued Effectiveness of COVID-19 Vaccination among Urban Healthcare Workers during Delta Variant Predominance) tracked vaccinated and unvaccinated Massachusetts healthcare workers and showed 0 infections in 74,557 person-days for previously infected patients compared to 49 infections out of 830,084 person-days for fully vaccinated patients.
Summing it up:
In short, vaccine mandates that don’t exempt those who have recovered are unethical and a danger to the health of society. They are preventing us from getting to “herd immunity” which we can achieve through allowing natural infection and treating with effective early treatment protocols. The study also concluded that the vaccine efficacy was 76.5% (95% CI: 40.9–90.6%) against Delta. Yet other data shows the vaccines do nothing or make things worse. I didn’t see an obvious flaw in this study regarding that determination. I don’t know if they used different Ct values for vaccinated or unvaccinated. If anyone sees a flaw, please comment below. SummaryThis study adds more evidence that recovered immunity >> vaccine immunity. Even if the vaccines were perfectly save, forcing everyone to get vaccinated is both unnecessary and jeopardizes public health. Even if I ignore all the other data sources and only believe this one small study, it doesn’t change my opinion on the safety of these vaccines. DO NOT GET VACCINATED. You are always better off getting COVID, getting early treatment as soon as you have symptoms (safer and more effective than any vaccine), and then you are done. This is what Aaron Rodgers did. He maximized benefits for himself, his teammates, and society. Win-win-win. |
Abstract
Background Data on COVID-19 vaccine effectiveness (VE) among healthcare workers (HCWs) during periods of delta variant predominance are limited.
Methods We followed a population of urban Massachusetts HCWs (45% non-White) subject to epidemiologic surveillance. We accounted for covariates such as demographics and community background infection incidence, as well as information bias regarding COVID-19 diagnosis and vaccination status.
Results and Discussion During the study period (December 16, 2020 to September 30, 2021), 4615 HCWs contributed to a total of 1,152,486 person-days at risk (excluding 309 HCWs with prior infection) and had a COVID-19 incidence rate of 5.2/10,000 (114 infections out of 219,842 person-days) for unvaccinated person-days and 0.6/10,000 (49 infections out of 830,084 person-days) for fully vaccinated person-days, resulting in an adjusted VE of 82.3% (95% CI: 75.1–87.4%). For the secondary analysis limited to the period of delta variant predominance in Massachusetts (i.e., July 1 to September 30, 2021), we observed an adjusted VE of 76.5% (95% CI: 40.9–90.6%). Independently, we found no re-infection among those with prior COVID-19, contributing to 74,557 re-infection-free person-days, adding to the evidence base for the robustness of naturally acquired immunity.
Background
Data on COVID-19 vaccine effectiveness (VE) among healthcare workers (HCWs) during periods of delta variant predominance are limited. Literature accounting for other potential determinants of infection rates is even more scarce.
Objective
To investigate the continued effectiveness of COVID-19 vaccination during the Delta variant predominance in a diverse and urban healthcare setting.
Methods and Findings
A community-based healthcare system in Massachusetts runs a COVID-19 vaccination program for employees (described previously (1)), with the Pfizer vaccine starting on December 16, Moderna on December 23, 2020, and J&J/Janssen in February 2021. Vaccination was available to all workers regardless of their in-person/remote working status from December 29, 2020. In addition, the system announced a vaccine mandate on August 16, 2021, which requires employees to receive their final dose by October 18, 2021 barring an approved religious or medical exemption.
We followed all actively serving HCWs in the system from December 16, 2020 to September 30, 2021, excluding those with prior COVID-19 infection from the main analyses. The outcome was having a positive PCR assay during the study period documented by the healthcare system’s Occupational Health department (2). The established master database, comprised of workers’ demographics, prior infection, de novo PCR positivity, vaccination (validated by the Massachusetts Immunization Information System and/or the healthcare system’s medical records), and human resource administrative data, has been previously described (1, 2). For each HCW, we calculated the person-days at risk and categorized them according to vaccination status. A HCW’s follow-up person-days were censored at the end of the study period, his/her termination date, the date tested positive for COVID, or the date he/she received a 3rd vaccine dose, whichever came first.
The Andersen-Gill extension of the Cox proportional hazards models were built to account for correlated data. We further adjusted for age, sex, race, and the Massachusetts statewide 7-day average of tested COVID cases (3) on the date the first dose was given to control for background rates. We estimated VE by calculating 100%×(1−hazard ratio). The R software (version 3.6.3) was used for statistical analyses.
A total of 4,615 HCWs (average age of 45.0±13.3 years and female predominance (76.0%)) contributed to 1,152,486 person-days at risk during the study period. Forty-five percent of the study population was non-White (including 20% African American, 13.5% Hispanic, and 9.0% Asian). Of all HCWs, 4,418 (95.7%) had received at least one dose by the end of the study. Among them, 58.3% got Moderna, 39.4% Pfizer, 2.3% J&J/Janssen, and one (0.02%) got mixed doses of J&J/Janssen and Moderna. The results showed that throughout the study period, for fully vaccinated HCWs the VE is 82.3% (95% CI: 75.1–87.4%) after multivariable adjustment (Table, Figure).
Table.Rate of infection during the study period (Dec 16, 2020 – Sep 30, 2021) across the four vaccination categories (excluding 309 people infected before Dec 15, 2020)
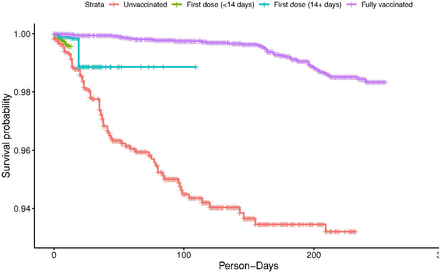
Figure.The Kaplan-Meier curve for the survival (i.e. infection-free) person-days across the four categories based on vaccination status
We further conducted a secondary analysis limiting the study period from July 1, 2021 to September 30, 2021, corresponding to delta variant predominance in Massachusetts (4). We observed an incidence rate of 5.8/10,000 (15 events out of 25,910 person-days) for unvaccinated person-days and 1.3/10,000 (39 events out of 308,267 person-days) for 14 days after fully vaccinated, resulting in an adjusted VE of 76.5% (95% CI: 40.9–90.6%).
When we examined HCWs (n=423) with infections occurring before vaccination, no re-infection was observed, accumulating 74,557 re-infection-free person-days (starting 10 days after initial infection and censoring at the date of receiving their first vaccine dose). Further, after vaccination, previously infected HCWs did not contribute any breakthrough infection events among the vaccinated HCWs.
Discussion
To our knowledge, this study is one of the first in healthcare settings regarding continued VE during delta variant predominance. Our work also provides further evidence of naturally acquired immunity. We found similar VE against the delta variant, 76%, compared to another study’s findings, 66% (5). Strengths included accounting for covariates and information bias such as demographics and background incidence, a multiethnic study population, consistent COVID-19 screening criteria, and well-validated vaccination records. Nonetheless, we did not examine individual manufacturers’ VE due to a limited power.
Footnotes
-
Conflict of interest disclosure: S.N.K. has received COVID-19-related consulting fees from Open Health and has owned shares of Regeneron, Moderna and Astra-Zeneca. All other authors declare no competing interests.
-
Financial support: None reported.