A growing wave of lawsuits is challenging the safety warnings accompanying blockbuster weight-loss drugs that have rapidly reshaped American medicine and culture, raising questions about whether patients were adequately informed of the risks tied to medications now used by tens of millions of people.
The plaintiffs’ stories vary widely, but share a common claim: that drugs known as GLP-1 receptor agonists – including Ozempic, Wegovy and Mounjaro – caused severe, life-altering injuries that were not sufficiently disclosed at the time they were prescribed.
A Maryland truck driver says he suffered what doctors described as an “eye stroke,” losing vision first in one eye and then the other. A Louisiana woman developed a serious neurological condition after weeks of vomiting and malnutrition. An Oklahoma real-estate agent alleges her colon ruptured without warning while she was driving her granddaughter home from a softball game.
“My colon blew up. Literally blew up,” said JoHelen McClain, the Oklahoma plaintiff. “I was trying to slim down and feel healthy.”
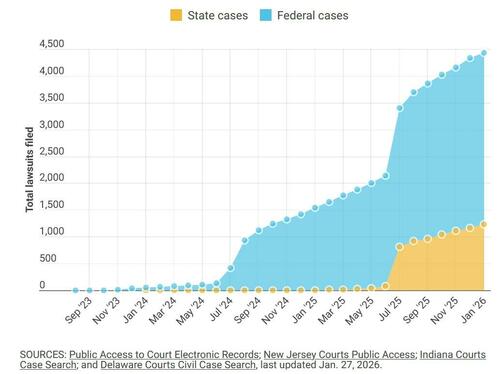
All three are among more than 4,400 plaintiffs who have filed lawsuits since 2023 against the drugs’ manufacturers, Novo Nordisk and Eli Lilly, according to court filings. The cases are now consolidated into federal and state litigation expected to take years to resolve.
The suits come amid explosive growth in the use of GLP-1 drugs. An estimated 12% of American adults – more than 31 million people – are currently using a GLP-1 medication, according to the nonpartisan health policy group KFF. Prescriptions rose from roughly one million in 2018 to about nine million in 2022, and usage doubled again between 2024 and 2025, Gallup data show.
Originally developed to treat diabetes, the drugs mimic a hormone that slows digestion, stimulates insulin release and increases feelings of fullness. Their success has helped reduce U.S. obesity rates for the first time in more than a decade and spurred research into additional benefits, including reduced risks of kidney disease, addiction and dementia.
Yet plaintiffs allege that the same mechanism slowing digestion can, in some patients, lead to serious gastrointestinal and neurological injuries.
In court on Jan. 13, Novo Nordisk attorney Katie Insogna said (via USA Today):
-
75% of the federal lawsuits include an allegation of gastroparesis, also known as “stomach paralysis,” a chronic condition where the stomach slows or stops emptying food into the small intestine;
-
18% of the cases allege the drugs caused ileus, a condition in which bowel muscles fail to push food and waste out of the body;
-
18% of the plaintiffs allege intestinal obstructions;
-
8% say they suffered from gallbladder injuries, with some of these patients requiring surgical removal of gangrenous tissue;
-
8% of the plaintiffs allege other serious gastrointestinal complications, such as extreme vomiting, chronic acid reflux or abdominal pain that required multiple hospitalizations in some cases. Others say their digestion issues have continued even after they stopped taking the drugs.
USA TODAY’s review of the lawsuits also found at least 110 plaintiffs alleging sudden blindness or severe vision changes, and at least one alleging Wernicke’s encephalopathy, a neurological condition linked to vitamin B1 deficiency.
The drugmakers deny the allegations. In a joint filing last year, the companies said “the safety profile of GLP-1 RAs has been well-established in hundreds of clinical trials, large-scale observational studies, and nearly two decades of real-world use.”
“Novo Nordisk remains confident in the benefit-risk profile of our GLP-1 medicines, when used consistent with their indications and product labeling,” said company spokesperson Flavia Brakling, adding that labels are updated “in cooperation with the FDA and consistent with federal regulations.”
An Eli Lilly spokesperson said, “Patient safety is Lilly’s top priority,” noting that the labels for Mounjaro, Zepbound and Trulicity have “always warned of potential ‘gastrointestinal adverse reactions, sometimes severe.’”
Legal experts say the cases may hinge on whether plaintiffs can prove causation and whether warnings were legally sufficient at the time.
“Proving the drugs caused certain outcomes will be an issue,” said Ana Santos Rutschman, a health-law professor at Villanova University, along with determining “the extent and timing of warnings.”
For Todd Engel, the Maryland truck driver, the outcome has already been devastating. After four months on Ozempic to manage diabetes, he woke in December 2023 with vision loss in one eye. Diagnosed with non-arteritic anterior ischemic optic neuropathy, or NAION, he continued taking the drug after doctors failed to identify a cause. In October 2024, he lost vision in his remaining eye.
“You’re not going to believe this,” his wife, Shelley, recalled him saying. “I can’t see at all.“
Now legally blind, Engel has lost his job and commercial driver’s license. “This whole thing has been catastrophic to me and my wife,” he said.
A 2024 JAMA Ophthalmology study of nearly 17,000 patients found an increased risk of NAION among those prescribed semaglutide compared with other treatments, though it did not establish causation. European regulators later described the condition as “very rare,” prompting label updates abroad. U.S. labels warn of vision changes but do not mention NAION by name.
“What happened to me should have never happened,” Engel said.
McClain’s case unfolded differently. After losing 40 pounds on Wegovy, she suffered a sudden colon rupture in March 2024, followed by emergency surgery, sepsis and months of recovery. She now lives with a permanent stoma.
“I read everything I could find on it before I went on it,” she said. “They did not warn about any of the stuff that happened to me at that time.”
In Louisiana, Mark Smith says his wife Robin developed permanent brain damage after months of vomiting while taking Mounjaro. Doctors later diagnosed her with Wernicke’s encephalopathy.
“I still have my wife, physically, not mentally anymore,” he said.
Eli Lilly has said Mounjaro’s label has always warned of severe gastrointestinal reactions. Plaintiffs argue those warnings failed to convey how extreme or irreversible some outcomes could be.
“These drugs are not new,” said Ziyad Al-Aly, director of research at the St. Louis Veterans Affairs Health Care System. “What’s new about them is that the companies then realized, ‘Oh my God, they actually work on weight loss.’”
Al-Aly said he sympathizes with the plaintiffs but believes the drugs’ benefits outweigh the risks for most patients. “There is nothing that’s really all benefit and no risk,” he said.
The first bellwether trials in the consolidated litigation are not expected until 2027. Legal experts say such cases often take four to five years.
Loading recommendations…